The BioBeat Chest Patch or sensor is one of a new generation of wearable physiological monitors that has become available in the last few years. It is a regulated clinical device and Incarta was the first company to register the device with the Therapeutic Goods Administration (TGA) for sales in Australia.
What does it measure
The BioBeat chest patch is an enhanced photoplethysmographic (PPG) sensor that uses machine learning to derive the major vital signs used for sub-acute patient monitoring. It is special because of the range of measurements it is capable of and for its low cost of approximately $140. In summary, the sensor measures:
- Heart rate
- Respiration rate
- Oxygen saturation
- Blood pressure (systolic, diastolic)
- Skin temperature
- Systemic vascular resistance
- Stroke volume
- Cardiac output
- Cardiac index
- Single lead ECG
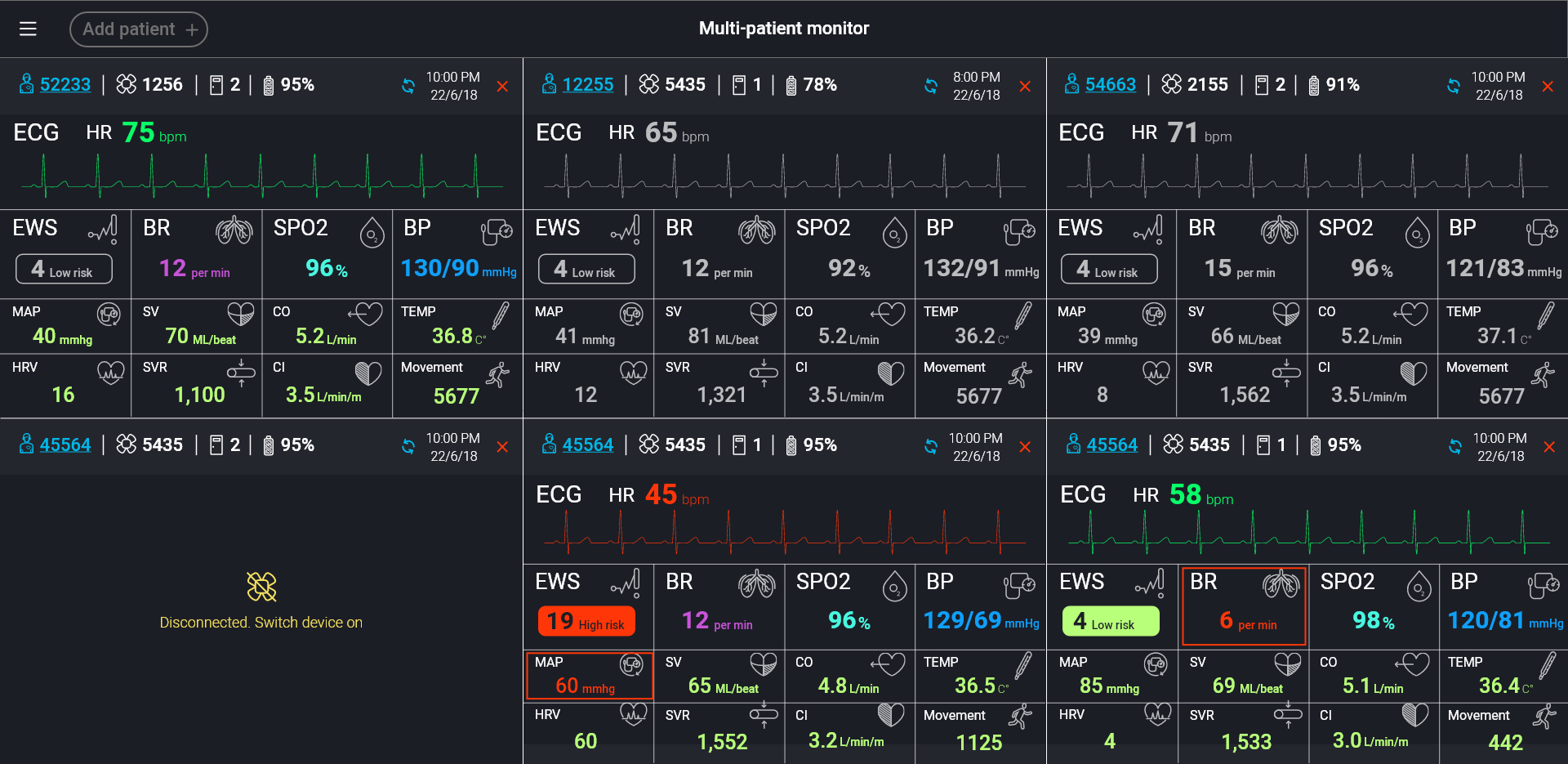
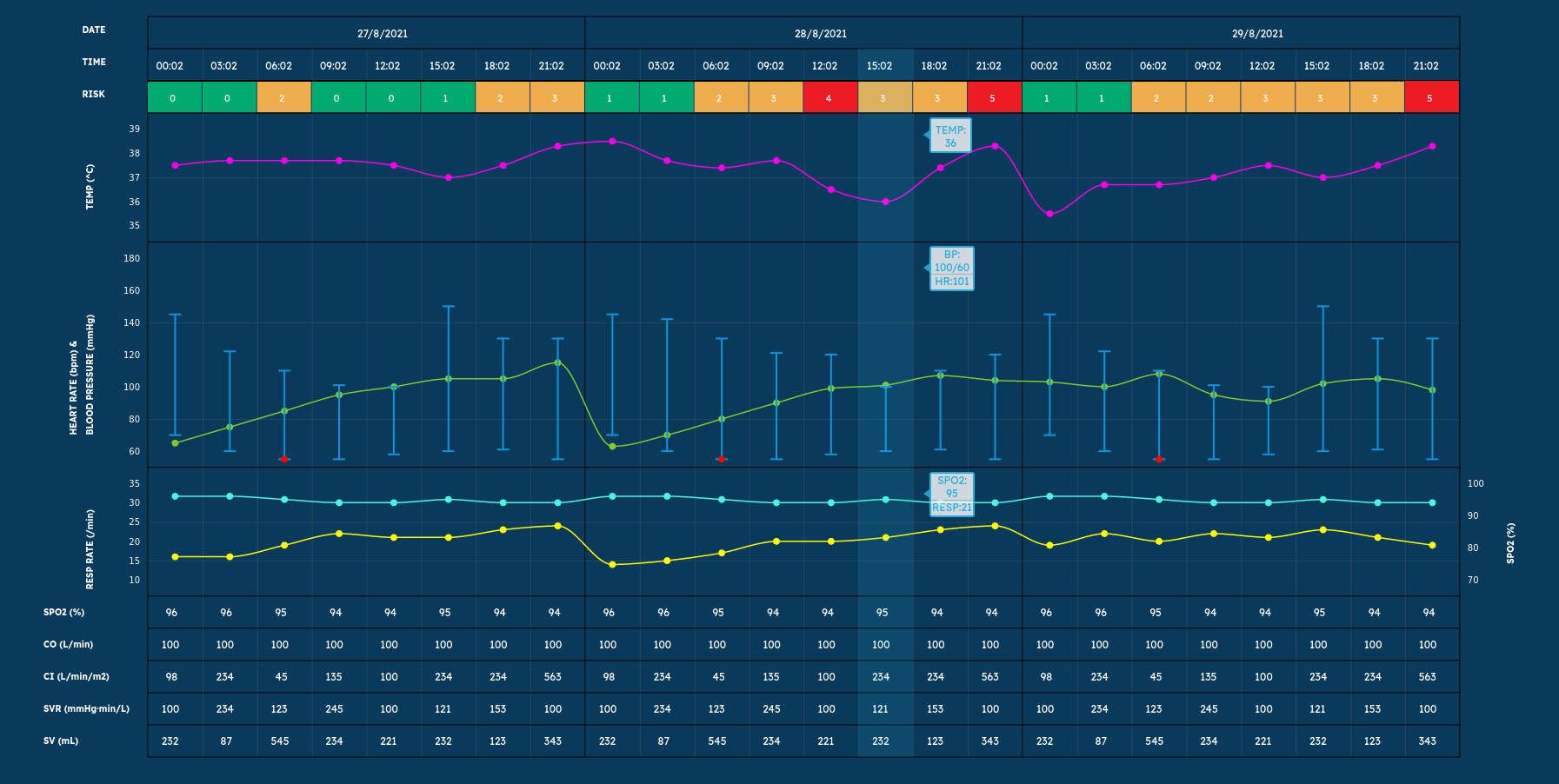
The data is viewable in a portal that looks much like a patient monitor. We have included an example in this post to give you an idea of sophistication of the technology.
Ease of use
The sensors are extraordinarily easy to use. They come with adhesive unit made from material much like an ECG electrode. The adhesive is pressed onto the chest and the device clipped in and turned on. For in-patients, BioBeat recommends the use of fixed gateways. These are small “black boxes” much like a router that are permanently connected to the hospital network. The fixed gateways enable the sensors to automatically connect to the real-time portal. When using a mobile phone gateway an extra step is required to connect the sensor to the phone. Once connected, the sensor serial number is entered into the portal together with some basic reference information like patient blood pressure and heart rate and the data starts flowing.
COVID-19
The BioBeat sensor is a wireless device and therefore particularly useful for monitoring of COVID-19 patients both in the home and in hospitals. We recently ran some trials and in this setting and thought it would be useful to detail some of our experience.
The sensor is an effective monitor and the early warning scores appear to be useful in identifying at risk patients. One of the immediate advantages that was identified for in-patient monitoring was the reduction in nursing contact time. Once the device is in place it can remain there for 24 hours (before the adhesive needs to be replaced).
It is an interesting exercise to consider the cost benefit of these sensors. A day in a ward of tertiary hospital costs in the order of $1500. Intensive care unit (ICU) the costs are closer to $4000 per day. In addition to the obvious benefits to the patient from a care perspective, if an at risk patient can be identified earlier and appropriate management initiated then transfer to a hospital or in fact from a ward to ICU may not be required with a consequential cost saving.
During the trial, sensors were placed on SCOVID patients as they arrived in emergency. These sensors remained in place for confirmed COVID patients who were admitted and and transferred to the COVID ward. Patients who were considered well enough to be managed at home were sent home with a sensor linked to their mobile phone. They could then be monitored remotely by the clinical staff and regularly assessed for clinical status. The vital signs of all these patients were viewable on the real-time portal.
Lessons learnt
Like all technology there are number of considerations that will make your life easier if you choose to use these devices.
Position and skin preparation
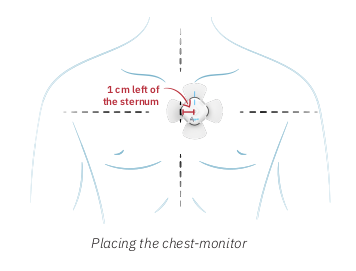
It is critical to locate the device precisely in order to get stable, reliable readings. The user guide has very clear instructions and they should definitely be followed (see the diagram on the right)! The area of the chest where the device is to be placed should be shaved and cleaned with an alcohol swab. Again missing this step may result in sub-optimal measurements. We found that it was important to replace the adhesive every 24 hours in order to minimize skin irritation and suggest that each time it is replaced the skin is again cleaned with an alcohol swab.
Gateways
Sensors cannot be easily moved from a mobile gateway to a fixed gateway of vice-versa. Start where you plan to finish. We also found numbers of patients with limited data plans on their mobiles; after the plan ran out, the data stopped transmitting! We even had patients turning their phones off or forgetting to charge them. Of course the real-time portal will help you to identify these issues so you can notify the patients concerned.
Data
Data retention is an important consideration when dealing with patient information and keeping records of the data that drives clinical decision making is a legislated requirement in Australia. The BioBeat portal offers several features that help to maintain compliance. First is the option of printing daily reports. These reports must be printed each day as there appears to be a limit on printing historic records. More importantly, once a patient is discharged from the real-time portal data is lost. However, BioBeat also offers an API as an add-on. This enables the raw data to be downloaded for maintenance of clinical histories and research. However, the downloaded data is not in a form that is immediately usable and a health service would need access to programming and / or data analytics resources to build interfaces to their electronic medical record systems. To this end, Incarta has developed an HL7 interface that can merge the wearable data with enterprise medical record systems. We also have a full wearable EMR platform for recording, viewing and analyzing wearable vital signs. Incarta’s Alarta EMR can merge full patient demographics, episode status, pathology, consolidated multi-day wearable charting, clinical notes and interventions into the wearable data set enabling a health service to maintain compliance and deliver an effective level of care.